Our Science
Trishula is dedicated to improving the outcomes for people with cancer. TTX-030 is a first-in-class antibody that inhibits the activity of CD39. TTX-030 targets the ATP-adenosine pathway and inhibition of CD39 is believed to modulate immune suppression within the tumor microenvironment, thus increasing anti-tumor immunity.
Pipeline
The clinical development plan for TTX-030, a novel immunotherapy, is focused on restoring and bolstering immune responses in the tumor microenvironment.
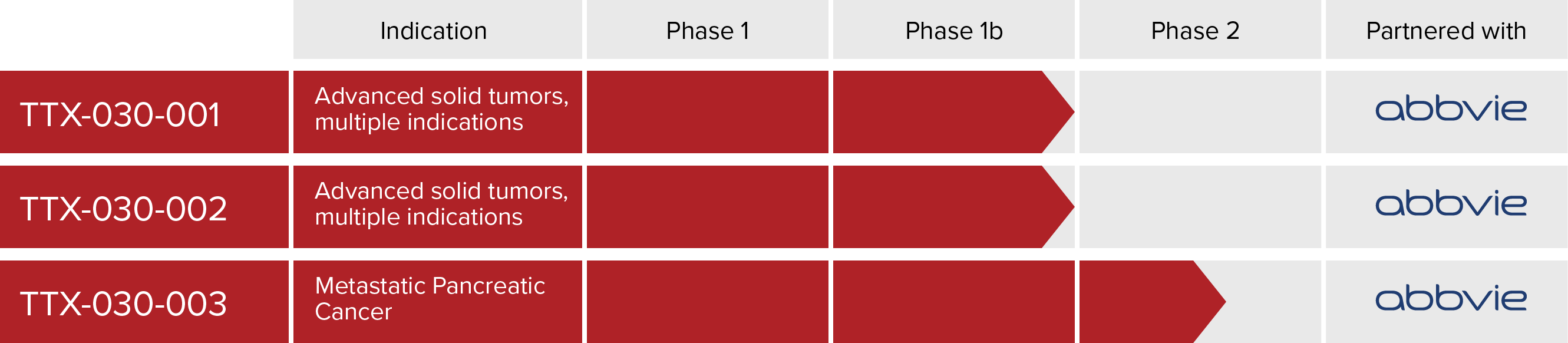
See clinical trials: TTX-030-001 (NCT03884556), and TTX-030-002 (NCT04306900) and TTX-030-003 (NCT06119217)
ATP and Adenosine in the Tumor Microenvironment

Tumors employ various strategies to create an environment that reduces the immune system’s ability to detect and fight cancer.
The ATP-adenosine pathway plays a key role in establishing an immunosuppressive tumor microenvironment (TME) by driving the conversion of proinflammatory, extracellular ATP to immunosuppressive adenosine. Trishula’s TTX-030 aims to reverse this process in two ways:
- By inhibiting the production of adenosine in the TME, TTX-030 prevents the adenosine-mediated inhibition of immune effector cells (including T cells, B cells, NK cells and myeloid cells).
- By maintaining high levels of extracellular ATP, TTX-030 enables the stimulation of dendritic and myeloid-derived cells necessary to support innate and adaptive immunity.
This dual mechanism of action is designed to reverse the immunosuppressive conditions within the TME and restore the immune system’s anti-tumor capabilities.
TTX-030, A NOVEL, FIRST-IN-CLASS, ANTI-CD39 ANTIBODY

TTX-030 is an antibody that inhibits the activity of CD39, the rate-limiting enzyme in the conversion of ATP to adenosine in the tumor microenvironment.
TTX-030 is being studied in phase 1/1b clinical trials as a monotherapy and in combination with anti-PD-1 immunotherapy and standard chemotherapy in adults with advanced cancer (NCT03884556 and NCT04306900)
TTX-030 is being studied further in a phase 2 clinical trial in combination with standard chemotherapy, with or without budigalimab (an investigative anti-PD-1 antibody) vs standard chemotherapy in first line metastatic pancreatic cancer (NCT06119217)
Publications
Zev Wainberg et al. Combination Treatment with TTX-030, a First-in-class Anti-CD39 Antibody, in Patients with Advanced Pancreatic Cancer. ESMO 2024
Zev Wainberg et al. Safety and efficacy of TTX-030, an anti-CD39 antibody, in combination with chemoimmunotherapy for the first line treatment of locally advanced or metastatic gastric/GEJ cancer. Cancer Res (2022) 82 (12_Supplement): CT015.
Moesta AK, Li XY, Smyth MJ. Targeting CD39 in cancer. Nat Rev Immunol. 2020; 20(12):739-755.
Spatola B et al. Fully human anti-CD39 antibody potently inhibits ATPase activity in cancer cells via uncompetitive allosteric mechanism. mAbs. 2020; 12(1):1838036.
Yan J et al. Control of metastases via myeloid CD39 and NK cell effector function. Cancer Immunol Res 2020; 8(3):356-367.
Li XY et al. Targeting CD39 in Cancer Reveals an Extracellular ATP- and Inflammasome-Driven Tumor Immunity. Cancer Discovery. 2019; 9(12):1754-1773
